We are
biotech
innovators

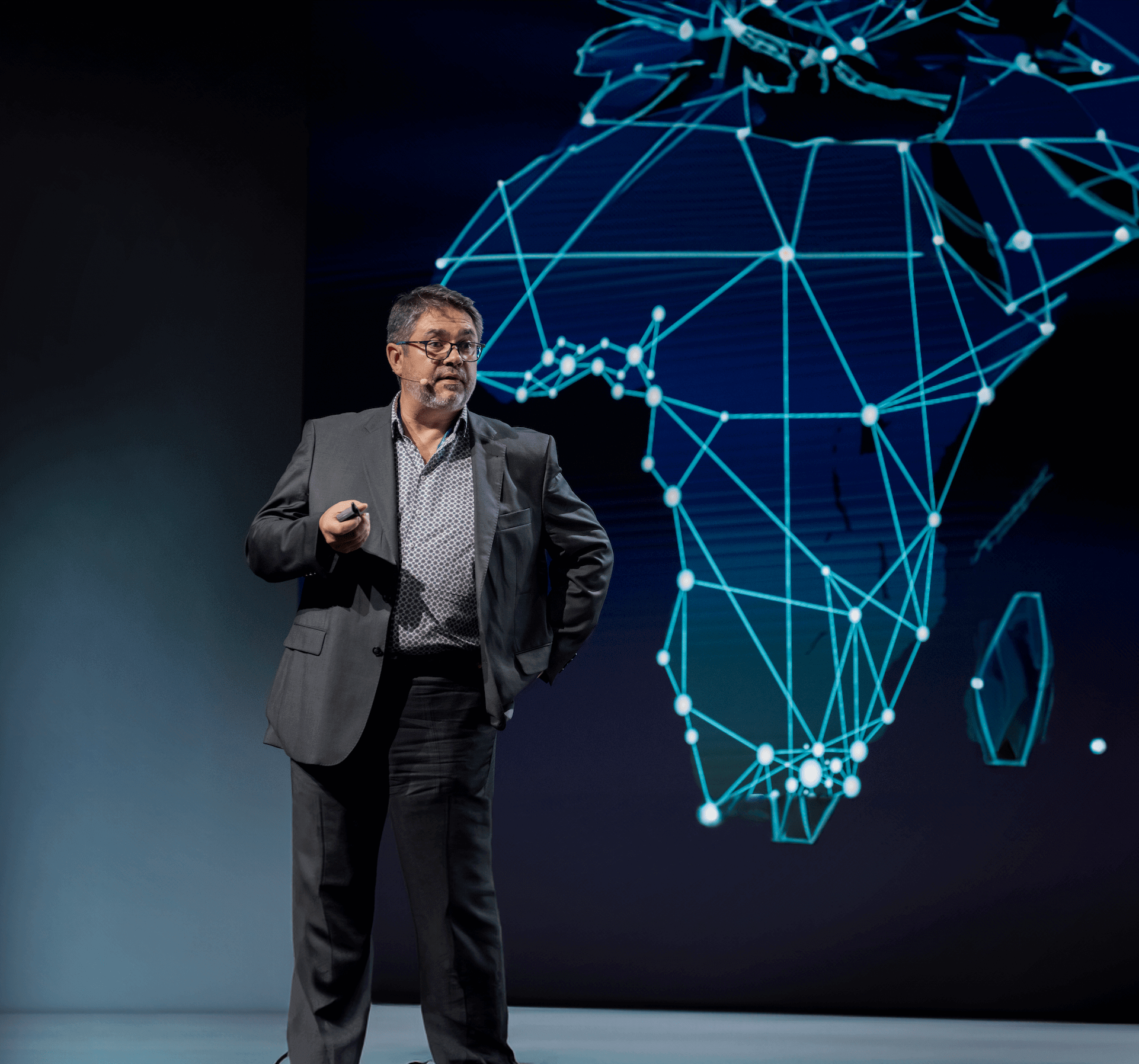
We are a global group of biotech innovators, rooted in Belgium, driven by one purpose: To transform how biotech drugs are made so everyone, everywhere, can get them.
Through cutting-edge bioprocessing and technology, we create transformative processes and platforms across drug discovery, development, and delivery.



Innovation at Univercells

Sustainability

At Univercells, sustainability isn’t an afterthought; it’s woven into our operations. Sustainability is a balance between maximizing societal impact, ensuring financial sustainability, and aligning with our Environmental, Social, and Governance commitments.
Download our 2023 Impact Report
“The innovative team we have built to support Univercells’ purpose is the real source of the value we create.”
Vincent Vanderborght, CFO
Join Us
Stakeholders
As scientists and entrepreneurs, we generate value by bringing market-driven innovation to life.
We embrace challenges. Where others see roadblocks, we find a way around them to create unique businesses. Our shareholders understand that, with a strong dynamic of long-time supporters welcoming new investors throughout various equity rounds.
Our diverse pool of stakeholders has played a crucial role in our successes, challenging us to go faster and further.